Author’s Note: This article, “GLP-1 Alternatives After Compounding Shutdown”, should not be taken as medical instruction. Always consult a professional for personal health decisions.
The Shutdown of GLP-1 Compounding Pharmacies: Now What?
Patients who relied on compounded GLP‑1 injections are now scrambling for GLP‑1 alternatives after compounding shutdown. Over the past year, soaring demand for brand GLP‑1 drugs (Ozempic/Wegovy/tirzepatide) led many to seek cheaper compounded versions. For the background on how these drugs work in the body, see our extensive article “How GLP-1 Works: Understanding the Science”.
A recent ADA guidance notes that high use of these weight‑loss drugs caused “intermittent shortages, thus prompting…compounded formulations” ADA. Now, with key court rulings and FDA decisions, the era of compounded semaglutide and tirzepatide is over FDA ADA. Many patients feel abandoned and unsure where to turn next.

Patients are seeking GLP-1 alternatives after compounded versions were shut down. Discover what options remain.
Rise of Compounded GLP‑1 and Legal Battles
In 2023–2024, GLP‑1 prescriptions surged as these drugs gained fame for diabetes and obesity. As brand‑name supplies lagged demand, out‑of‑pocket costs pushed patients toward compound pharmacies. For example, one study found dozens of compounding businesses advertising unapproved semaglutide or tirzepatide weight‑loss injections, citing access barriers and high prices PubMed. The ADA notes this trend: “increasing utilization…has contributed to intermittent shortages, thus prompting multiple entities to produce and market compounded formulations…directly to consumers.” ADA.
Pharmaceutical companies challenged the compounders. By early 2025, courts sided with the manufacturers and the FDA. In February 2025, the FDA declared the semaglutide shortage resolved FDA, and in May 2025 a judge similarly ended compounding of tirzepatide FDA FDA. As a result, compounding pharmacies must stop selling their knock‑off GLP‑1 injections, leaving patients without these low‑cost alternatives.
The Current Crisis: Patients Left Behind

Patients face rising costs for GLP-1 meds after compounding shutdowns. 54% of users already struggle with affordability.
Now, many patients describe feeling anxious, frustrated and left behind. Having relied on affordable compounded shots, they find themselves abruptly cut off. A Kaiser poll found that even insured users struggle: 54% report difficulty affording GLP‑1 drugs, with most paying a large share of the cost AJMC.
In plain terms, patients fear weight regain and uncontrolled blood sugar without their familiar therapy. They often feel the system favors those who can pay for brand‑name treatments. The ADA has issued guidance for providers on managing medication shortages ADA, but many individuals still have urgent questions. For instance, insurance coverage for alternatives can be confusing.
According to one analysis, GLP‑1 list prices range roughly $936–$1,349 per month before insurance AJMC. Many people worry that with compounding gone, only the expensive FDA‑approved versions will remain. The gap in care is real: “as demand continues to increase, it is possible that intermittent shortages may occur,” the ADA noted, emphasizing the need for guidance when medications are unavailable ADA.
Exploring Liraglutide (Victoza/Saxenda)
An important approved alternative is liraglutide, sold as Victoza (for diabetes) and Saxenda (for weight management). Liraglutide is a GLP‑1 receptor agonist – it mimics the natural GLP‑1 hormone, which stimulates insulin after meals and helps curb appetite Mayo Clinic. Unlike Ozempic/Wegovy, liraglutide is injected once daily. Patients typically start at 0.6 mg and ramp up to 1.2–1.8 mg (Victoza) or up to 3.0 mg (Saxenda) per day, depending on the use.
In clinical trials, liraglutide/Saxenda produced modest but meaningful weight loss. About one‑third of patients on Saxenda lost over 10% of their body weight after one year, compared to under 7% on placebo FDA. (In one study, Saxenda users averaged ~33.9% achieving >10% loss vs 15.4% on placebo FDA.) Liraglutide also improves blood sugar control and has heart‑ and kidney‑protective benefits in diabetes management.
🔥Hot Tip🔥
💡 Fun Fact 💡
Compared head‑to‑head, liraglutide’s weight‑loss effect is smaller than semaglutide’s. For example, semaglutide 2.4 mg (Wegovy) yields roughly twice the weight reduction of liraglutide 3.0 mg (Saxenda) PMC. However, liraglutide remains valuable because it is FDA‑approved and widely prescribed.
Victoza has been used for diabetes for years, so many insurers cover it for blood sugar management (though coverage varies for weight loss). Saxenda (the weight‑loss brand) is more expensive and may not be covered unless strict criteria are met.
Other GLP‑1 and Related Medications
The table below compares available GLP‑1 (and related) drugs:
| Medication | Brand(s) | Form | Use | Approx. Weight Loss |
|---|---|---|---|---|
| Liraglutide | Victoza (diabetes), Saxenda (weight) | Daily injection | Type 2 diabetes; obesity | ≈5% |
| Semaglutide | Ozempic (diabetes), Wegovy (weight), Rybelsus (oral) | Weekly injection; daily pill | Type 2 diabetes; obesity | ≈12% (Wegovy dose) |
| Tirzepatide | Mounjaro (diabetes), Zepbound (weight) | Weekly injection | Type 2 diabetes; obesity | ≈20%+ |
| Dulaglutide | Trulicity | Weekly injection | Type 2 diabetes | ≈3–5% |
| Exenatide | Byetta (BID), Bydureon (weekly) | BID or weekly injection | Type 2 diabetes | ≈2–4% |
| Oral Semaglutide | Rybelsus | Daily pill | Type 2 diabetes | ≈3–4% |
The table shows that semaglutide (Ozempic/Wegovy) and tirzepatide produce the largest average weight loss PMC. Liraglutide and dulaglutide also help with weight, though to a lesser extent. Exenatide and oral semaglutide have smaller but still better weight‑loss effects than no treatment. For more on these drugs, see our Ozempic vs Wegovy guide.
Patients on compounded GLP‑1 may also consider other FDA‑approved weight‑loss drugs (though not GLP‑1s) such as bupropion/naltrexone (Contrave), phentermine/topiramate (Qsymia), or orlistat (Alli/Xenical). Some of these have moderate effects and different side effect profiles. We have written extensively about the side effects of semaglutide and tirzepatide in our article “The GLP-1 Side Effects No One Talks About”. A Story of SGLT2 inhibitors (like Jardiance or Farxiga) are another class for diabetes that can aid weight loss modestly.
Taking Action: What You Can Do Now
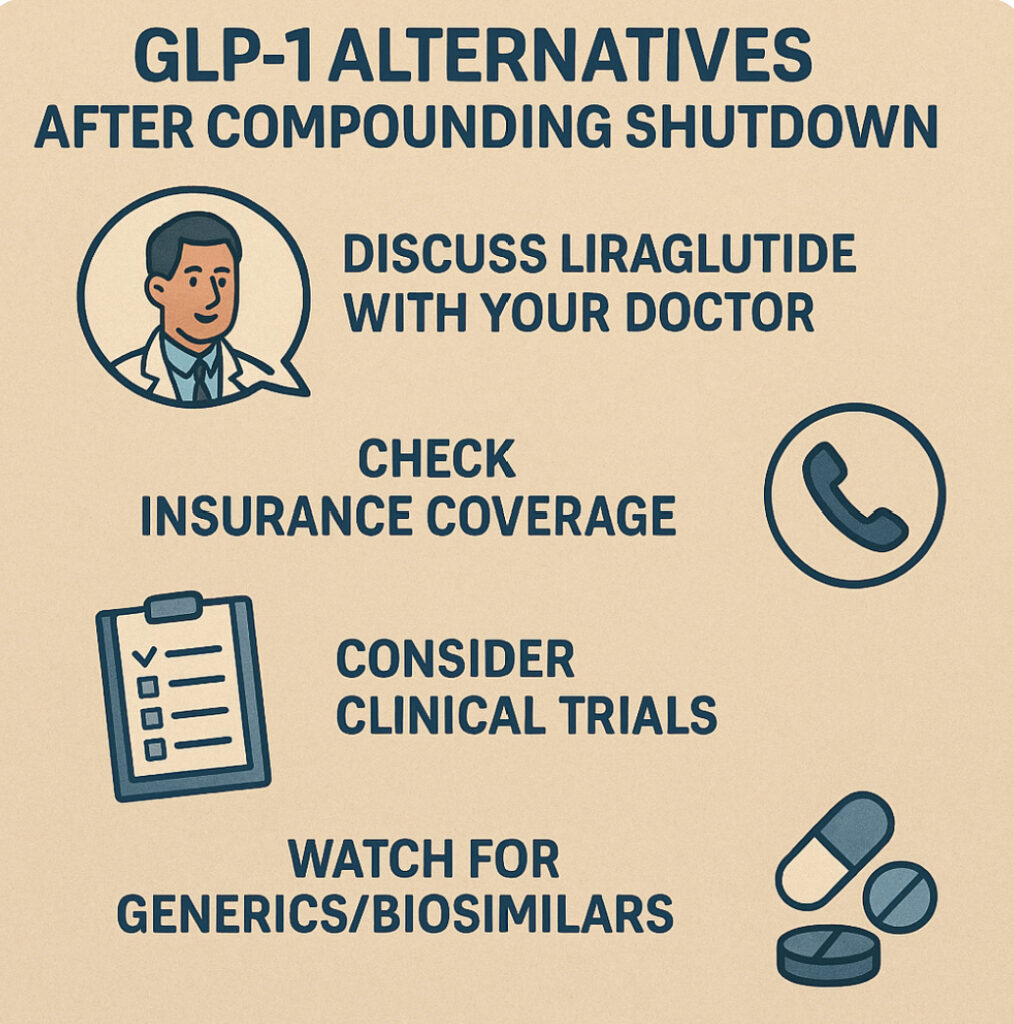
If you’ve lost access to compounded semaglutide/tirzepatide, here are steps to take:
- Discuss Liraglutide with Your Doctor: Ask whether Victoza or Saxenda could replace your previous therapy. Doctors can prescribe these FDA‑approved GLP‑1s and advise on dosing.
- Check Insurance Coverage: Call your insurer or employer health plan. Confirm coverage for Victoza/Saxenda under diabetes or obesity benefits. Enroll in any available copay assistance. Even if you had used compounding before, the plan may cover these branded drugs with a medical diagnosis.
- Consider Clinical Trials: New GLP‑1 therapies (and combinations) are being tested. Ask your doctor about local trials or visit ClinicalTrials.gov for studies on obesity and diabetes drugs.
- Watch for Generics/Biosimilars: Multiple companies are developing semaglutide biosimilars. U.S. patents for Ozempic/Wegovy expire around 2032, but international generics could arrive in the mid‑2020s Columbia University. Stay informed about new approvals that could lower costs soon.
Remember, you are not alone. Many healthcare providers are now helping patients navigate this new landscape.
Patients face rising costs for GLP-1 meds after compounding shutdowns. 54% of users already struggle with affordability.
Looking Ahead: Hope and Innovation
Although the compounding shutdown is a major upheaval, it doesn’t mean progress has stopped. FDA‑approved GLP‑1 therapies remain available, and more are coming. Companies worldwide are racing to develop long‑acting and combination therapies. For example, dozens of researchers are working on even more potent GLP‑1/GIP combinations (like tirzepatide) and other novel hormones. Generic and biosimilar versions of semaglutide are expected in upcoming years Columbia University.
In short, this is a setback – but only a temporary one. Modern diabetes and obesity care are evolving rapidly. By working closely with your doctor and staying informed, you can still find an effective treatment plan. Progress in GLP‑1 therapies continues apace, and new, affordable options will emerge. Stay hopeful: there are still many paths forward for managing weight and diabetes health.
Disclaimer: This article is a personal narrative intended for informational purposes. It is not medical advice. Consult a healthcare professional for guidance on weight loss or any medical treatment. Also, note that Claire Aldington is a fictional character created to illustrate real experiences.
WORKS CITED
ADA. statement on compounded incretinsADA
FDA. GLP‑1 supply stabilizationFDA
STAT News: GLP‑1 pricing analysisSTAT News
KFF. survey on GLP‑1 affordabilityKFF
Mayo Clinic: Liraglutide overviewMayo Clinic
JAMA. SCALE trial (liraglutide 3.0 mg)JAMA
NEJM. STEP 1 trial (semaglutide 2.4 mg)NEJM
Lancet. Liraglutide CV outcomesLancet
PubMed. Knee load vs weightPubMed
NIDDK. NIDDK weight‑management guidanceNIDDK
Cleveland Clinic. Contrave: Cleveland Clinic explainerCleveland Clinic
BMJ. Qsymia review 2023BMJ
ClinicalTrials.gov: GLP‑1 trial searchClinicalTrials.gov
NIH: New incretin therapiesNIH
Harvard Health: GLP‑1 pipelineHarvard Health



